
What you need to know about biomarkers and biomarker testing.
In the context of stomach cancer, tumor-related biomarkers, as well as a person’s unique genetic biomarkers, have the potential to impact a patient’s diagnosis, prognosis and treatment. It is important to know whether a biomarker is driving your cancer and, if so, which one. Not all stomach cancers have the same biomarkers, and there are a variety of drugs available that may only be useful and available when certain biomarkers are present. Biomarkers can also change during the course of treatment.
Fill out this form for a downloadable script to bring to your next appointment.
Biomarker testing is relatively new, and some health care providers may not be up to date. In such cases, it is important to advocate for yourself and find a provider who is knowledgeable about biomarker testing, or to press your current provider on the issue. If you are unsure what to ask your health care team about biomarker testing, fill out this form for a downloadable script to bring to your next appointment.
Biomarkers can play a crucial role in tailoring targeted therapies and personalized treatment plans for stomach cancer patients. While new biomarkers are being discovered at a rapid rate, those listed below are among the most common biomarkers impacting stomach cancer treatment right now.
- HER2 (Human Epidermal Growth Factor Receptor 2): The HER2 gene makes HER2 proteins, which can make cancer grow. When stomach cancer cells contain a higher-than-normal amount of the HER2 protein, they can grow more quickly and spread to other parts of the body. These tumors can be treated with targeted antibody drugs.
- MSI-high and dMMR (Microsatellite instability high and mismatch repair deficiency): MSI-H or dMMR are not exactly the same thing, but are interrelated. The Mismatch Repair System is responsible for correcting errors that occur during DNA replication. It identifies and fixes mismatches or small loops of DNA called microsatellites. When the dMMR biomarker is present, it means the MMR system is broken, and can cause a high rate of genetic mutations in microsatellite regions. When someone is MSI-high, it means their DNA is prone to mutations. These biomarkers may be responsive to immunotherapy such as keytruda.
- PD-L1 (Programmed Death-Ligand-1): If tumor cells contain abnormally high amounts of a protein called PD-L1, targeted drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) can be used to prevent the suppression of T cells and free up the immune system to fight the cancer.
- TMB: (Tumor mutational burden): If cancer cells have more than one gene mutation, they are said to have a high TMB, which means they have a higher chance of responding to immunotherapy checkpoint inhibitors such as the drug pembrolizumab (Keytruda).
- EBV: Epstein-Barr virus may play a role in transforming normal stomach cells into cancerous ones.
- Tumor-agnostic biomarkers: Tumor-agnostic biomarkers are molecular characteristics that are related to many different types of cancer, including stomach cancer. These biomarkers, such as NTRK (Neurotrophic Tyrosine Receptor Kinase), BRAF mutations and RET fusion, are not limited to a specific organ or tissue, and are increasingly important in guiding targeted therapies for a variety of cancers, including stomach cancer. For example, while rare, NTRK tumors respond well to targeted therapy such as larotrectinib (Vitrakvi) and entrectinib (Rozlytrek).
Emerging biomarkers are biomarkers not yet widely established, but ongoing research suggests they have the potential to impact treatment plans and outcomes. There are a number of emerging stomach cancer biomarkers already beginning to factor into treatment plans, and patients may want to research and ask their care team about them.
- CLDN 18.2 (Claudin 18.2): Claudins are proteins that help control how molecules flow between cells. Claudin 18.2 is found in the stomach lining, and higher-than-normal levels of it are associated with stomach cancer. While research into Claudin 18.2 and stomach cancer is ongoing, there is reason to be optimistic about the use of monoclonal antibodies as a targeted therapy.
- FGFR2b (Fibroblast growth factor receptor 2b): The FGFR2 gene provides instructions for making a protein involved in the regulation of cell growth and division. FGFR2b is a “splice variant” or unusual form of FGFR2 that is associated with many diseases and disorders. FGFR2b is being explored as a biomarker for stomach cancer which could be targeted with antibodies in combination with chemotherapy.
- MUC17 (Mucin 17): Mucins are proteins that exist within a thin wall of skin that produces stomach-protecting mucous. MUC17 is found in higher-than-normal amounts in around half of gastric cancers, and a clinical trial exploring an immunotherapy called AMG 199 in treatment of MUC17-positive stomach cancer was recently concluded.
- KRAS (Kirsten rat sarcoma viral oncogene homolog): The KRAS gene determines the development of the KRAS protein, which helps control cell growth and division. A variant form of KRAS, which can cause uncontrolled cell growth and contribute to cancer development, can sometimes be found in many types of cancer, including stomach cancer.
- DKK1 (Dickkopf-1): The DKK1 protein helps regulate cell growth and development. An elevated DKK1 level is associated with stomach cancer, as well as other cancers.
What are common stomach cancer biomarkers?
HER2 (Human Epidermal Growth Factor Receptor 2)
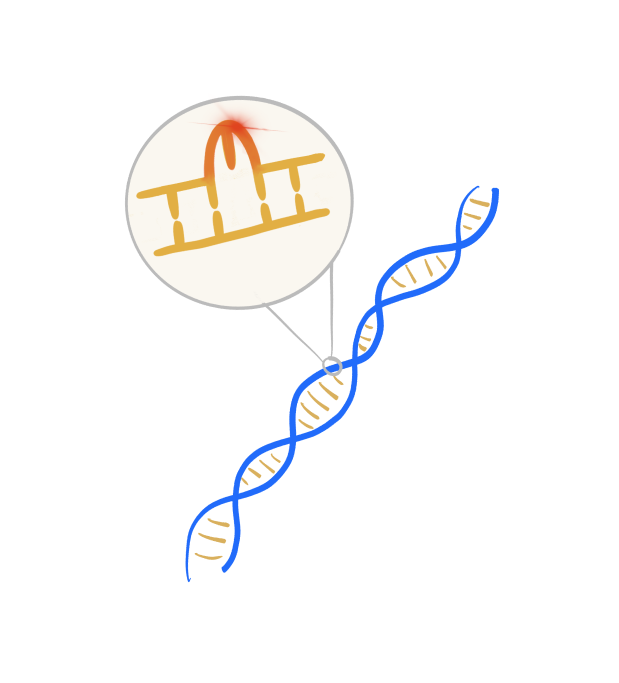
MSI-high and dMMR (Microsatellite instability high and mismatch repair deficiency)
PD-L1 (Programmed Death-Ligand-1)
If tumor cells contain abnormally high amounts of a protein called PD-L1, targeted drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) can be used to prevent the suppression of T cells and free up the immune system to fight the cancer.
TMB (Tumor mutational burden)
EBV (Epstein-Barr virus)
Tumor-agnostic biomarkers
CLDN 18.2 (Claudin 18.2)
Claudin 18.2 is found in the stomach lining, and when cancer develops, claudin 18.2 may become more exposed on the cancer cell surface. The Food and Drug Administration (FDA) approved zolbetuximab (Vyloy), a monoclonal antibody targeting claudin 18.2, in combination with chemotherapy for the treatment of adult patients with locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma that are claudin 18.2-positive.
What are emerging biomarkers, and why are they important?
FGFR2b (Fibroblast growth factor receptor 2b)
DKK1 (Dickkopf-1)
The DKK1 protein helps regulate cell growth and development. An elevated DKK1 level is associated with stomach cancer, as well as other cancers.

